Vol. XXXII Issue 1
Article 2
DOI: 10.35407/bag.2021.32.01.02
ARTÍCULOS ORIGINALES
Ex situ plant germplasm
conservation revised at the light of mechanisms and methods of genetics
Conservación de germplasma Ex situ revisada
a la luz de mecanismos y métodos de genética
Camadro E.L.1 *
Rimieri P.2
1 Consejo Nacional de Investigaciones Científicas y Técnicas
(CONICET) and Facultad de Ciencias Agrarias, Universidad Nacional de Mar del
Plata (FCA, UNMdP), Ruta Nacional 226 km 73.5, 7620 Balcarce, Argentina.
2 Estación Experimental Agropecuaria Pergamino, Instituto
Nacional de Tecnología Agropecuaria (INTA), Avenida Frondizi (Ruta 32) km 4.5, 2700
Pergamino, Argentina.
* Corresponding author: Elsa L. Camadro camadro.elsa@inta.gob.ar
ORCID 0000-0002-1739-5059
ABSTRACT
Plant genetic resources for food and agriculture are ex situ conserved
in germplasm banks as samples (accessions) of natural or naturalized
populations, either as the originally sampled propagules (mainly seeds) or
their multiplications. The premises underlying ex situ conservation are
that (a) it is the safest and cheapest alternative for germplasm preservation
for future generations and (b) accessions are representative of the genetic
diversity encountered in nature. In the past decades, ideas, alternatives and
considerations have been put forward on the topic, and protocols have been
devised for plant germplasm sampling, conservation and multiplication. However,
limitations in the management efficiency of germplasm banks have been pointed
out by international organizations. In our opinion, germplasm banks in general
need to revise their functioning and management at the light of principles and
methods of Genetics. To that end, it is necessary to consider the reproductive
biology of higher plants -whose genetic consequences at both the individual
plant and the population levels are not always either fully understood or taken
into account in devising the protocols-, the genetic structures of wild and
cultivated populations, and the course of the genetic material in the
populations. In this paper, we discuss the three topics and provide an example
of a national forage breeding program, from germplasm bank accessions as the
germplasm of origin to the obtainment of commercial cultivars. Finally, we
present a proposal as a base for discussion among curators, researchers and
breeders.
Key words: Accessions, Breeding, Genetic resources, Germplasm
banks, Population genetics
RESUMEN
Los recursos genéticos vegetales para la
alimentación y la agricultura se conservan ex situ en bancos de
germoplasma como muestras (introducciones) de poblaciones naturales o
naturalizadas ya sea como propágulos originales (mayoritariamente semillas) o
sus multiplicaciones. Las premisas subyacentes son que (a) es la alternativa
más segura y barata de preservación de germoplasma para futuras generaciones y
(b) las introducciones son representativas de la diversidad genética que se
encuentra en la naturaleza En las últimas décadas, se han presentado ideas,
alternativas y consideraciones sobre el tema y se han elaborado protocolos para
el muestreo, conservación y multiplicación de germoplasma. Sin embargo,
organizaciones internacionales han señalado limitaciones en la eficiencia del
manejo de los bancos de germoplasma. En nuestra opinión, se necesita revisar el
funcionamiento y manejo de dichos bancos en general a la luz de los principios
y métodos de Genética. Para tal fin, es necesario considerar la biología reproductiva
de las plantas superiores -cuyas consecuencias genéticas a nivel de planta
individual y de población no se comprenden en su totalidad o no se consideran
al idear los protocolos -, las estructuras genéticas de poblaciones naturales y
cultivadas, y el curso del material genético en las poblaciones. En este
trabajo discutimos los tres temas y proveemos un ejemplo de un programa
nacional de mejoramiento de forrajeras, desde las introducciones como
germoplasma de origen hasta la obtención de cultivares comerciales. Finalmente,
presentamos una propuesta como base de discusión entre curadores,
investigadores y mejoradores.
Palabras clave: Introducciones, Mejoramiento
genético, Recursos genéticos, Bancos de germoplasma,
Genética de poblaciones
Received: 06/15/2021
Accepted: 06/23/2021
INTRODUCTION
With the aim of contributing to the development of coherent and
effective strategies for conservation of plant genetic resources for food and
agriculture, ideas, alternatives and considerations have been put forward over
the years in many methodological publications. Limitations in the management
efficiency of germplasm banks, not infrequently carried out without appropriate
planning, were pointed out in “The State of the World´s Plant Genetic Resources
for Food and Agriculture” (FAO, 1996). In that report, it was considered that
over 65% of the worldwide ex situ conserved collections needed
regeneration. Almost 10 years later, the logistics of germplasm banks was
integrally analyzed in the last manual published by Biodiversity International
(previously IBPGR or International Board for Plant Genetic Resources) (Engels
and Visser, 2006). As judged by the magnitude of the advancements made over the
previous decades at the global level, the authors recognized that the response
of germplasm banks had been scarce regarding the utilization of the appropriate
strategies for the ex situ conservation of collections. For curators,
this manual constituted a guide for adopting a more critical, balanced and
creative approach to germplasm conservation. Useful information was presented
on various management aspects to solve frequently encountered operative
problems with the incorporation of new and better technologies. In particular,
important elements were analyzed and options were discussed to improve the
efficiency and effectiveness of operations both according to costs and by
taking into account genetic and economic implications for rationalization of
the logistics. From a further analysis of the history and evolution of
germplasm banks, it was concluded that these banks had gone through periods of
questioning about their function or operativity. Among others, the following
reasons were given: limited resources; excess or loss of accessions; lack of
representativeness of the natural genetic diversity in the accessions, modifications
in conservation and multiplication protocols, and changes in the conservation
objectives due to the demands of breeding (development of commercial varieties)
and agroecological programs (preservation of local varieties or landraces).
More than a decade has gone by since the publication of Engels and Visser´s
(2006) document. However, in our opinion, there is still a need to revise the
functioning and management of germplasm banks in general. We consider that it
is timely to present an approach at the light of principles and methods of
Genetics. In this regard, the principles and methods established and used at
the individual level (cell, tissue, organ, organism) (e.g., what is the genetic
material, how it is transmitted and arranged, how it changes and functions) are
not the same as those established and used at the population level (which are
related to the course of the genetic material in the populations). We consider
that our proposal -based on considerations of the modes of reproduction and
their genetic consequences, the genetic structures of wild and cultivated
populations, and principles of population genetics- could serve as a base
document for discussion among curators, researchers and breeders on the
adequacy of the current protocols for ex situ conservation of the
natural genetic diversity. To the best of our knowledge, this approach relating
gametes, gene flow, fertilization and other biological phenomena that have
important genetic components has not previously been integrally and routinely
used. In this regard, there are many examples in the literature in which the
“structure” of collections of wild or cultivated species has been assimilated
to the “genetic structure” of populations or in which the term has been used in
regard to the total genetic diversity and its partitioning at various levels by
means of statistical analysis (ANOVA, AMOVA, STRUCTURE program), even though
the definition of “genetic structure” in Genetics is clearly different, as it
will be discussed. Moreover, for some statistical analyses (e.g., traditional
cluster analysis) it has been considered appropriate to assume that sexual
reproduction can occur either by autogamy or allogamy and, therefore, that a
population of an autogamous species is genetically homogeneous and a population
of an allogamous species is genetically heterogeneous. However, the variability
that can be encountered in the genetic structure of a natural population at a
given time would depend, among other factors, on the preponderant mode and type
of reproduction of the population of origin, as it will be explained.
Ex situ CONSERVATION
Plant germplasm conservation is mainly carried out ex situ in the
form of samples of propagules (accessions). These propagules can be either the
originally sampled ones in natural or naturalized populations, or their
regenerants obtained in the same bank or from interbank exchange. In the last
decades, there has been a change in emphasis away from this type of
conservation and towards the in situ conservation of locally adapted
landraces and crop wild relatives (CWR) within or outside protected areas (Maxted et al., 1997; Maxted et al.,
2016; FAO, 2017). However, ex situ conservation has advantages and
disadvantages per se and in relation to other conservation methods
(Kjaer et al., 2001, in Hammer and Teklu, 2008); thus, the ex situ
and in situ approaches are complementary, fulfilling different
purposes. Plant accessions are usually conserved under specific categories,
mainly assigned according to morphological phenotypes, with the relatively more
recent incorporation of molecular tools (see Camadro, 2012). This type of
classification into taxonomic or typological species (TS) responds to the
Taxonomic Species Concept (TSC); according to this concept, species are
immutable entities because they have reached the end of the evolutive process.
Plants can also be classified as biological species (BS) on the basis of
breeding relationships when the Biological Species Concept (BSC) is applied,
regardless of their morphological phenotypes. TS and BS do not necessary
overlap; thus, the use of the term ‘‘species’’ generates much confusion when
the distinction between them is not clearly made (see Grant, 1981). Moreover, taxonomic
categories are periodically subjected to revision because they are human
constructions. Thus, taxonomic nomenclatures and “species” numbers in a given
plant group can vary over the years according to the taxonomist(s) involved in
the task. For example, the number of potato “species” (Solanum L.
section Potato; Dicotyledoneae) has been reduced in the last 40 years from
approximately 235 (seven of them cultivated and 228 wild) to 203, 189 and 111
(four of them cultivated and 107 wild) (in Poulsen Hornum
and Camadro, 2021), whereas in brome grasses (Bromus L. section Ceratochloa),
with approximately 160 recognized “species”, the large morphological variation
encountered in the section led Williams et al. (2011) to point out that
“Hybridization is rife in this section, making species boundaries obscure and
the taxonomy very difficult”. Notwithstanding, and as previously stated,
collections are assigned specific categories for their incorporation and
conservation as accessions in germplasm banks, without specification of the concept
(either TSC or BSC) used for their classification (see an example at http://www.ars-grin.gov/npgs/collections.html).
The species concept employed in the taxonomy of a plant group, however, has
genetic consequences for both conservation and seed regeneration and
multiplication protocols (see Poulsen Hornum and Camadro, 2021).
Germplasm bank accessions can be composed of (a) seeds of sexually reproducing
or apomictic plants; (b) plants derived from vegetative organs (e.g., tubers,
stolons, corms, leaves) cultivated in the field, or plantlets cultivated in
vitro; (c) pollen, embryos or tissues conserved in liquid nitrogen (FAO,
2017). This type of conservation is justified when: (a) natural or
naturalized populations are subjected to -or at risk of being subjected to-
genetic erosion, or are affected by the extinction of native or naturalized
plant communities; (b) there is a need for developing or complementing breeding
programs through pre-breeding in less domesticated species, or for
complementing working collections in breeding programs of advanced-breeding
species for transferring genes or gene combinations from unexploited sources;
(c) there are lines, clones or compounds synthesizing general adaptation,
agronomic aptitude and productive potential that have been discarded in
breeding programs, or varieties of reference that have been replaced by new
ones in the commercial circuit but that can eventually be of value in breeding;
(d) there are landraces or old varieties, often linked with traditional food
products and organoleptic properties, that have cultural or economic value (or
both) for small farmers.
¿WHAT PART OF THE
GENETIC DIVERSITY NEEDS TO BE PROTECTED?
Ex situ conservation steps from the premises that (a) this form of conservation
is the safest and cheapest alternative for preserving plant genetic resources
for forthcoming generations, and (b) accessions are representative of the
diversity encountered in the environments from which they were sampled: spatial
(landscape, plant communities), morphological, and molecular. The two
premises -along with the provision of detailed passport information- are
important. However, an approach is needed to ensure that accessions faithfully
represent both the sampled populations and the portion of the genetic diversity
that needs to be protected. It has to be taken into account that genetic drift
can occur if, in planning the operations, there is not a strict consideration
of a combination of various phenomena. These can span from manipulations at the
sampling time to various aspects of reproductive genetics during seed
regeneration or multiplication, including the possible action of internal
crossing barriers within accessions, e.g., male sterility, pollenpistil
incompatibility, nuclear-cytoplasmic genome interactions, among other
biological phenomena (see Camadro 2012; Poulsen Hornum
and Camadro, 2021). Thus, the estimation of genetic diversity ought to
be complemented with detailed information on the genetic structure and
reproductive biology of the population at the sampling time and, fundamentally,
during the ex situ regeneration or multiplication processes. This last
concept, if not integrally applied, nullifies the premise of security,
economics and representativeness of the accessions because duplicates would not
be detected and some gene (allele) frequencies might be unknowingly increased,
decreased or eroded during the multiplication process. In summary, the genetic
diversity and variability represented by an accession could be unnoticedly
changed during propagule regeneration or multiplication; as a consequence, the
accession would no longer represent the actual diversity and variability of the
sampled population (Hammer and Teklu, 2008; Erazzú et al.,
2009; Cadima et al., 2017; Poulsen Hornum and Camadro, 2021).
GERMPLASM BANKS
Many germplasm banks had their origin in plant breeding and research
programs and were not necessarily designed to assimilate genetical approaches
for in situ and ex situ conservation. Thus, it is important to
critically examine the precise objectives of germplasm banks to identify
possible limitations in their functioning. If clear objectives are established,
it would be feasible to plan what genetic resources should be conserved and to
choose the most adequate protocols for that end, establishing priorities and
recognizing limitations and the biological complexities of the species of
interest, including the form of propagation., Frankel (1984) proposed to establish
core collections to facilitate germplasm management and use after defining the
objectives. Core collections are collections of limited size, with minimum
similarity among the composing accessions and much smaller than the
collection(s) from which they were derived. Or as defined by Johnson and Hodgkin (1999), a core collection is
a subset of one collection that represents with minimum repetition the genetic
diversity of a cultivated species and its wild relatives.
A CONSERVATION APPROACH
BASED ON THE GENETIC STRUCTURE OF POPULATIONS
The main objective of ex situ conservation is to have the maximum
genetic diversity of a species represented in the accessions, previous
establishment of the necessity of conservation, the increment of the number of
propagules, and the maintenance of this diversity for conservation and
exchange. These aspects ought to be known to define the representativeness of
the originally sampled population in the accession. As complements, gaps and
priorities have to be identified in the collection for conservation of
strategic genetic resources and the determination of their potential applied
value.
GENETIC MAKEUP OF
POPULATIONS AND INDIVIDUALS IN NATURE
From a biological perspective, a natural population is a community of
potentially inter-breeding individuals growing at a given locality, which share
a common gene pool and represents a dynamic panmictic unit (Johansen 1903 and
Dobzhansky, 1935, in Rieger et al.,
1976). The largest group of potentially inter-breeding individuals is the
species which, in turn, is composed of local populations, each of them
inter-communicating and inter-grading with the others. The sum of all factors
governing the pattern by which gametes of various individuals unite with each
other during fertilization makes up the population structure which, in nature,
is a consequence of gene flow rates and environmental heterogeneity (Gilmoure
and Gregor, 1939, in Rieger et al., 1976). By extension, the genetic
structure of a population, either natural or artificial, is the type, quantity
and distribution of the genetic variation present in that population expressed
in terms of gene (allele) or genotypic frequencies. Thus, the genetic structure
of a population depends on the mode and type of reproduction of the plant group
or species that conform it. In this regard, it has to be taken into account
that higher plants can reproduce either sexually or asexually, or have both
types of reproduction available to them; consequently, the genetic structure of
a given population can vary over time.
MODES OF REPRODUCTION
AND GENETIC CONSEQUENCES
Sexual Reproduction
The production of sexual propagules (sexual seeds) entails the formation
of n megaspores and n microspores (pollen grains or male
gametophytes) by meiosis, followed by the formation of n female gametes
and n male gametes by post-meiotic mitosis. The double fertilization of
the n egg cell and the binucleated (n + n) central cell of
the female gametophyte (embryo sac), each by one of the two n male
gametes carried by the microspore, originates one 2n cell and one 3n cell
which, respectively, give rise to the 2n embryo and the 3n endosperm
by mitosis (Dumas and Mogensen,
1993). The events involved in sexual reproduction allow for the occurrence
of two rounds of genetic recombination: (1) at meiosis, by segregation of
chromosomes and genes, and (2) at fertilization, by nuclear fusion of the
uniting gametes. Therefore, each sexual cycle provides the opportunity for the
formation of new genotypic combinations.
Autogamy and allogamy
There are two types of sexual reproduction: allogamy or
cross-fertilization and autogamy or self-fertilization. Allogamy maintains
heterozygosity at most loci if the breeding population is large enough, whereas
strict self-fertilization leads to homozygosity in most loci and, eventually,
to allele fixation. Two main factors promote allogamy: spatial and temporal
separation of sexual organs. Spatial separation can occur (a) within the plant
itself, e.g. maize (Zea mays L.), which bears female and male
inflorescences at different positions along the axis, and (b) between plants,
e.g. asparagus (Asparagus officinalis L.), with individual plants
bearing only one type of imperfect flowers, either with stamens or pistils
(occasionally, perfect flowers are formed in either type of plant, allowing
self-fertilization). Temporal separation (dichogamy) is the result of
differences in the maturation time of female and male reproductive organs
(protogyny and protandry, respectively), which in a plant can occur in (a)
flowers or inflorescences along the axis, e.g. maize, or (b) within an
inflorescence, e.g. carrot (Daucus carota L.) and sunflower (Helianthus
annuus L.). However, there could be simultaneous maturation (homogamy)
without autogamy in the presence of other factors: (a) chasmogamy (the flower
is open when pollen is shed and/ or the stigma is receptive) in otherwise
cleistogamous flowers (the pollen is shed and the stigma is receptive when the
flower is closed), e.g. Bromus spp. section Ceratochloa (Wolff et al., 1996; Langer and
Wilson, 1965; Leofanti et al.
2013); (b) hercogamy (physiological barriers), in plants with genetically
controlled selfincompatibility systems in which the flowers are either (b1)
homomorphic (of one morphological type), e.g. potatoes and tomatoes (Solanum
L. spp.), stone fruits such as almonds and cherries (Prunus L.
spp.), Crucifers (Brassica L. spp.) such as cabbage, colza and kale,
among others, or (b2) heteromorphic, e.g. common flax (Linum usitatissimum L.)
and loosestrife (Lythrium junceum Banks & Sol.); and (c) sterility
(being male sterility the most frequent type) due to malformations in the
reproductive organs or abnormalities in meiosis that prevent either production
of viable pollen or its release from the anthers and, thus, self-fertilization.
Breakdown of hercogamy, dichogamy, or self-incompatibility precedes the shift
of the breeding system from obligate outcrossing towards autogamy due to
structural and positional changes in the hermaphrodite flower, bud pollination
and, finally, cleistogamy (in Frankel and Galun, 1977). Autogamy and
allogamy have both specular positive and negative characteristics. The positive
characteristics of autogamy vs. allogamy are: genotype fixation and
genotype specialization, which result in thriving of adapted genotypes over
time in stable environments; guaranteed fertilization with economy of pollen;
and adaptation to long distance dispersal because only one seed can start a
population. The negative characteristics of autogamy are the other face of the
coin: genetic inflexibility due to a lower capacity of “genetic storage” (of
alleles and intra-locus and inter-loci interactions) and, thus, inability of
the population to cope over time with changing environments (“evolutive
compression”); and unguaranteed fertilization with the consequent waste of
pollen.
Asexual Reproduction
Asexual propagules can originate by means of (a) seeds (agamospermy) or
(b) other structures (agamic or vegetative reproduction). In agamospermy, there
could be morphological alternation of generations or not. There is
morphological alternation of generations when diplosporous or aposporous 2n gametophytes
are formed, respectively, from 2n archesporial or 2n somatic
cells, and either the 2n egg or other 2n cell of the gametophyte
develops parthenogenetically in a process accompanied by the development of the
endosperm either after fertilization of the central cell (pseudogamy) or
without fertilization of this cell. On the other hand, there is no alternation
of generations if the 2n embryos develop by adventive embryony or
sporophytic budding from cells of the nucellus or integuments of the ovule
(somatic embryogenesis) (Asker, 1980; Burnham, 1980). In
plants with agamospermous reproduction, embryos (a) can be clones of the mother
plant if they originate by somatic embryogenesis, apospory, or diplospory with
a modified meiosis genetically equivalent to a mitosis, or (b) can genetically
differ from the mother plant if the modified meiosis in diplospory entails a
certain amount of recombination. In plants with agamic or vegetative
reproduction, propagules (bulbs, corms, tubers, stolons, or rhizomes, among
other structures) are formed by mitosis in somatic tissues, thus, they are
clones of the mother plant.
ARE THE MODES AND TYPES
OF REPRODUCTION STRICT?
Higher plants may have more than one mode or type of reproduction as a
result of genotype x environment interactions. Sexually reproducing plants can
be (a) autogamous, e.g. wheat (Triticum aestivum L.), tobacco (Nicotiana
tabacum L.), garden tomato (Solanum lycopersicum L.); allogamous,
e.g. maize, carrot, garden asparagus; (b) autogamous with a percentage of
allogamy, e.g. beans (Phaseolus L. spp.); (c) allogamous with a
percentage of autogamy, e.g. maize, sunflower (Helianthus annuus L.),
asparagus. Autogamous plants could be considered a prelude to evolutionary extinction
if it were not for the fact that local differentiation in ecological niches
maintains a massive storage of genetic diversity (in Frankel and
Galun, 1977). Similarly, asexual reproduction is not strict;
otherwise, it will also be an end road in evolution. It is frequently combined
with sexual reproduction by allogamy, e.g. potatoes, grasses.
NATURAL AND NATURALIZED
POPULATIONS
Sexually Reproducing
Species
In autogamous species, individual plants with disomic inheritance
(diploids and disomic polyploids, e.g. 2x Triticum monococcum L., 4x T.
turgidum L., 6x T. aestivum L.) are expected to be highly homozygous
for one genetic combination (Fig. 1a) or more than one (Fig. 1b). Populations of autogamous species, however, can be
genetically homogeneous to a greater or lesser extent depending on whether they
have a percentage of allogamy or not. For example, the percentage of allogamy
in Proso millet (Panicum miliacium L.), with wind-dispersed pollen, can
be more that 10%, whereas in Lima beans (Phaseolus lunatus L.), with
beesdispersed pollen, this percentage can range from 0% to 80%. Moreover, the
proportion of cleistogamous vs. chasmogamous flowers (e.g., in Lespedeza
Michx. ssp.) could variably increase the percentage of allogamy in a given
season (in Frankel and Galun, 1977). If individual plants
have opportunities for hybridization even from time to time, the population can
be composed of plants either homozygous for one genetic combination (Fig. 1a) or more than one (Fig. 1b), or heterozygous for
one or more loci (Fig. 1c) because they might
be F1 hybrids, backcrosses to the homozygous parents, or advanced segregating
generations. Therefore, populations can be either homogeneous or heterogeneous
in various degrees. In inbreeding species, the variation among populations is
expected to be larger than within populations in contrast with outbreeding
species. In a review of experiments carried out with isozymes in autogamous and
allogamous species, Schoen and Brown (1991) found that inbreeders
exhibited markedly greater population variation than outbreeders according to
Nei´s gene diversity statistics.
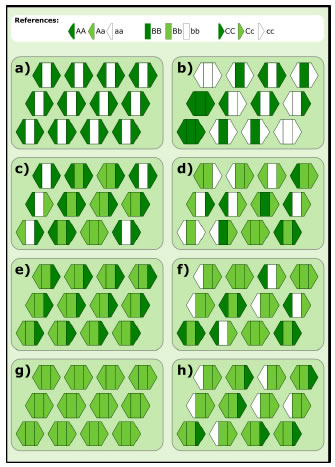
Figure 1. Genetic structure of
natural (NP) and breeding (BP) populations according to modes and
types of reproduction. NP: (a), (b) and (c) autogamous diploids and
disomic polyploids (a) homogeneous, with all loci in homozygosity in one
combination, (b) heterogeneous, with all loci in homozygosity in various
combinations, (c) with a percentage of allogamy; (d) allogamous diploids,
heterogeneous, with loci in homozygosity and heterozygosity; (e) to (h) clones,
homogeneous, with either loci in homozygosity and heterozygosity (e) or all
loci in heterozygosity (g) for one combination, or heterogeneous with more than
one genotype (f) and (h). BP: lines, homogeneous, with all loci in
homozygosity (a); F1 hybrids, homogeneous, with two loci in
heterozygosity (hybrid vigor) and one in homozygosity (overdominance) (e) and
with all loci in heterozygosity (g); populations, heterogeneous, of
autogamous (b) and allogamous (d) species; synthetics, heterogeneous,
with loci in homozygosity and heterozygosity (d); clones, homogeneous
with loci in homozygosity and heterozygosity in one combination (e) and (g), or
heterogeneous, with loci in homozygosity or heterozygosity in more than one
combination (f) and (h).
On the other hand, allogamy is obligate only in monoecious species with
strict self-incompatibility systems, and in dioecious species. The spatial and
temporal separation of the reproductive organs, as previously explained, promotes
but does not force this type of sexual reproduction. In individual plants of
both diploid and polyploid allogamous species, most loci are expected to be in
heterozygosity, although there could also be loci in homozygosity. Natural
populations are expected to be highly heterogeneous (Fig. 1d), being the genetic
diversity higher within than between populations as demonstrated, for example,
in wild potatoes (Bedonni and Camadro,
2009; Erazzú et al.
2009).
Asexually reproducing
species
A few higher plants exhibit only asexual reproduction (e.g. garlic, Allium
sativum L.) but most plants with this mode of reproduction can also
reproduce sexually under certain environmental conditions (see Frankel and Galun, 1977). The environmental
conditions can modify not only the proportion of allogamy in sexual reproducing
plants, as previously explained, but the preponderant mode of reproduction of a
given population as well. Examples can be found in apomictic grasses (Knox, 1967; Quarin, 1986; Rebozzi et al.,
2011) and wild potatoes (Leofanti et al.,
2019), among other plant groups. It is a common mistake to consider that
natural populations of asexually reproducing plants are genetically
homogeneous. On the contrary, these populations can be composed of plants of
either the same genotype (one clone; Fig. 1e and 1g) or different
genotypes (more than one clone; Fig. 1f and 1h) because
asexual reproduction is usually combined with sexual reproduction by allogamy.
Therefore, a population with the two alternative modes of reproduction can be a
mix of clones as a result of either hybridization followed by vegetative
reproduction in the subsequent generations or facultative apomixis. Individual
plants of asexually reproducing species can be highly heterozygous, but some
loci can be in homozygosis. Populations with asexual reproduction can be either
homogeneous or heterogeneous in various degrees (see Ellstrand and
Roose, 1987).
Summarizing, a thorough knowledge and understanding of the reproductive biology
and genetics of the species of interest is needed in order to (1) develop the
appropriate sampling and regeneration protocols to try to capture an important
amount of the genetic diversity present in a population, and (2) avoid or
minimize gene (allele) erosion during seed regeneration. Moreover, and given
that the types and modes of reproduction are not necessarily strict in a given
plant group and a given environment, it is: (a) inappropriate to carry out
statistical analyses under the assumption that populations have only one type
of reproduction (e.g. for sexually reproducing species, either autogamy or
allogamy) and, therefore, that they there are genetically either homogeneous or
heterogeneous, and (b) advisable to resample the populations in environmentally
contrasting years, whenever possible. In this regard, samples of a given
population taken in different moments should be used to conform the accession
(instead of naming each sample as a new accession) to maximize the amount of
the captured natural genetic diversity at a given site. It is our opinion that
no specific guidelines should be given for curators. Instead, and based on the
knowledge of the reproductive biology and genetics of the plant species or
group of interest, the principles and methods of population genetics should be
applied to prevent or reduce gene erosion in the conserved germplasm.
BREEDING POPULATIONS
Genetic makeup
Rimieri (2017) has pointed out that
it is necessary to differentiate ex situ and in situ conserved
plant genetic resources from those plant resources collected, maintained and
utilized for human subsistence, which are the result of the application of
selection or breeding methods. According to this approach, the protection of
the biodiversity and the application of mutagenic, biochemical, molecular and
genetic engineering tools are compatible and complementary. Plant breeding is
the heritable improvement of plants, usually acknowledged as a combination of
art and science. Approximately 11,000 years ago, domestication of plants and
animals evolved from the hunter-gatherer lifestyle. But it was in the 20th
century, with the rediscovery of Mendel´s laws of inheritance, that plant
breeding became an applied discipline, which makes use of principles from a
variety of other disciplines to improve the genetic potential of plants
cultivated for food, feed, and/or metabolites of interest, among others. Plant
breeders make use of conventional methods (parental selection, controlled
crosses, progeny selection) to introduce desirable traits to their object of
improvement (Gallais, 1990; Allard, 1999) with the relatively
more recent aid of biotechnologies, e.g., transgenesis, cisgenesis,
intragenesis, and gene edition (Al-Khayri et al.
2015; Cardi, 2016). In spite of the
advancements in genome manipulation, plant breeding remains a high time- and
resource-consuming process, particularly in crop species with narrow genetic
bases.
The final products of plant breeding are cultivated varieties or cultivars (a
term coined by contracting the two previous terms to establish a difference
with botanical varieties, which correspond to a taxonomic rank between
subspecies and form). Cultivars are obtained in usually long processes,
essentially Mendelian in nature and probabilistic. They are classified into
five types according to the reproductive system of the target species and the
genetic structure of the artificial populations: (1) lines or line
cultivar, generally of only one genotype (pure line; Fig. 1a); (2) F1 hybrid or hybrid cultivar, of
only one genotype resulting from a cross between two pure lines, with heterotic
effects, represented in Fig. 1e with two loci in
heterozygosity (hybrid vigor) and one locus in homozygosity (overdominance),
and in Fig. 1g with three loci in
heterozygosity; variants of this type of cultivar are named semi-hybrid
cultivars; (3) population or population cultivar, a mixture of
genotypes of either autogamous (Fig. 1b), allogamous (Fig. 1d), or apomictic plants. In forage crops, a population
cultivar composed of practically isogenic pure lines, similar in phenology and
morphological type, is known as a multiline cultivar; in allogamous
species, this type of cultivar is a population of wide genetic base resulting,
in general, from mass selection (Gallais and Bannerot, 1992); (4) synthetics
or synthetic cultivar, similar to population cultivars but only for
allogamous species, with paternal control of the origin (polycross) (Fig. 1d), or hybrids with low vigor depression in F2;
(5) clones or clone cultivar, composed of only one genotype (Fig. 1e), or two or more genotypes, e.g., clonal hybrids of
dioecious species such as asparagus (Fig. 1f) and scions and
grafts of fruit trees and ornamentals (Fig. 1h), selected from any
structure or obtained by mutagenesis and either macroor micropropagated
(Rimieri, 2017). The subject of the plant protection system -that will be
further explainedis a variety (cultivar), that is, a plant grouping within a
single botanical taxon of the lowest known rank. Such grouping is defined by
the expression of the characteristics resulting from either a given genotype
(e.g. one clone, line, or F1 hybrid) or a combination of genotypes (e.g., a
complex hybrid or synthetic variety) (UPOV, 2002).
INTELLECTUAL RIGHTS
PROPERTY
The conservation and utilization of plant genetic resources have always
required the consideration of diverse factors beyond the biological diversity itself.
Among others, the following can be mentioned: genetic transformation
technologies, technologies of information and communication (TICs), linked to
an increasing world recognition of the value of these resources (Visser and
Nap, 2002), and intellectual rights property of both genetic resources and
breeding products (Gepts, 2006). The International
Union for the Protection of New Varieties of Plants (UPOV) was created in 1961
to provide and promote an effective system of plant variety protection, with
the objective of encouraging the development new plant varieties in its
numerous member countries (UPOV, 2020). However, with the advent of plant
biotechnologies, patent rights began to affect the access to both genetic
resources and commercial varieties. In contrast to the breeder´s rights, patent
rights limit the access of third parties to patented genes, with the consequent
negative effect on the use of genetic resources. As Eriksson et al.
(2020) have discussed, different legal frameworks applicable to the use of
the genetic resources have been developed. With the scientific and technical
progress in research and breeding achieved in the past few decades, these
frameworks have become increasingly complex. Notwithstanding, the Convention on
Biological Diversity (CBD, 2020) in its art. 13, recognizes the sovereign
rights of the states on the genetic resources located within their frontiers.
Based on the principles contained in the CBD and the 2011 Nagoya Protocol plus
the decisions of the Parties, international goals on access and benefitsharing
have been established (see Sirakaya, 2019). UPOV is only
concerned with protected plant varieties. However, there is a spectrum of plant
genetic resources that does not fall into this category: populations of CWR,
landraces, and unprotected plant varieties. These genetic resources are not
affected by UPOV or plant breeders´ rights, but they may be regulated by other
treaties or schemes, e.g., the International Treaty on Plant Genetic Resources
for Food and Agriculture (ITPGRF), the previously mentioned CBD, and seed
marketing regulations (UPOV, 2016).
FROM GERMPLASM BANK
ACCESSIONS TO COMMERCIAL CULTIVARS
The potential utilization of ex situ conserved germplasm responds
to specific needs of broadening the genetic variability or the gene pool of the
breeders´ working collections, particularly in crop species in which the
advancements by selection are slow. From this germplasm, new genotypes or gene
combinations can be developed for incorporation into breeding programs (Cooper et
al., 2002; Rimieri and Wolff, 2010). One proposal to
combine a more efficient conservation of the genetic diversity present in the
accessions and to utilize part of the genetic variability of this germplasm in
plant breeding is the development of the previously mentioned core collections.
The establishment of core collections, which concentrate high genetic diversity
in a small number of samples with the avoidance of duplicates, can contribute
to the utilization of germplasm in research and pre-breeding, and to the
increase of the efficiency of germplasm bank management and interbank exchange.
Furthermore, with the complement of molecular biology tools, genetic
engineering and geographic information systems (GIS), the efficiency and
sustainable conservation of plant genetic resources advocated by FAO (1996)
would be likely incremented.
GENETIC RESOURCES,
POPULATION STRUCTURE AND OBTAINMENT OF COMMERCIAL CULTIVARS
The expansion of the genetic base and pre-breeding shortens the gap
between basic germplasm and crop genotypes. However, plant breeders seem to be
reluctant to employ plant materials coming directly from germplasm banks
because these materials lack, in general, adaptation for their use in breeding.
The lack of adaptation is a consequence of the cultivation environment of the
crop species and the agronomic management practices, plus the genetic
structures of commercial cultivars and the compatibility and interactions of
the wild germplasm with the genetic background of the breeder´s elite
collection. Notwithstanding, the three elements -genetic resources, population
structure, and commercial cultivar development- can be combined. Following, an
example is given of forage breeding program to illustrate the close
inter-disciplinary relationship between the use of germplasm from working collections
and germplasm banks and the application of methods and tools of commercial
cultivar development. In forage crops in general, cultivars are populations,
lines and genotypes adapted to the environmental and agronomic conditions of a
growing region. They may have their origin in one or more of the following: (a)
working collections of research groups involved in population evaluation and
selection, (b) foreign cultivars, (c) cultivars adapted to cultivation
conditions and animal utilization but no longer available in the market, (d)
breeders´ own collections obtained from native and naturalized populations or
from old implanted fields, and (e) selected samples -according to previously
defined criteria- from national and international collections of botanical
gardens, introduction and acclimatization gardens, and germplasm banks. It is,
therefore, necessary to remark that the decision on the germplasm to be
conserved and its possible utilization in breeding programs has to be based on
(1) the initial germplasm, obtained by collection or exchange, with special
emphasis in its representativeness of the genetic diversity of the species and
the adaptation to the environment and cultivation; and (2) consideration of (a)
agronomic and genetic parameters in the original samples and in the subsequent
characterization, (b) the predominant mode of reproduction, for propagule
multiplication, and (c) the predominant or more representative genetic
structures, also for propagule multiplication or the development of core collections,
pre-breeding, or commercial cultivar breeding.
TALL FESCUE AS AN
EXAMPLE
Tall fescue (Festuca arundinacea Schreb.) is a perennial forage
grass of temperate climate, of utmost importance and diffusion in Argentina.
This species is allogamous, with cleistogamous and chasmogamous flowers, of
hexaploid origin and with disomic inheritance. The breeding program carried out
at the Pergamino Experimental Station (Exp. Stn.), National Institute of
Agropecuarian Technology (INTA), in the Pampas region of central Argentina, is
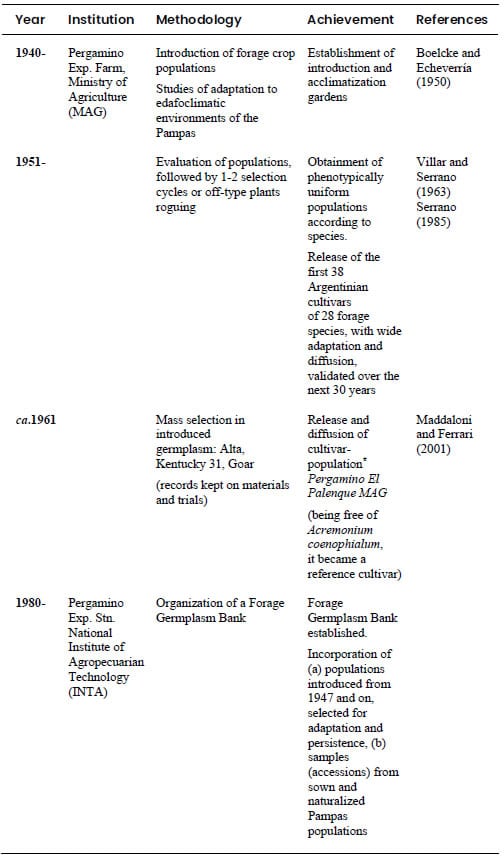
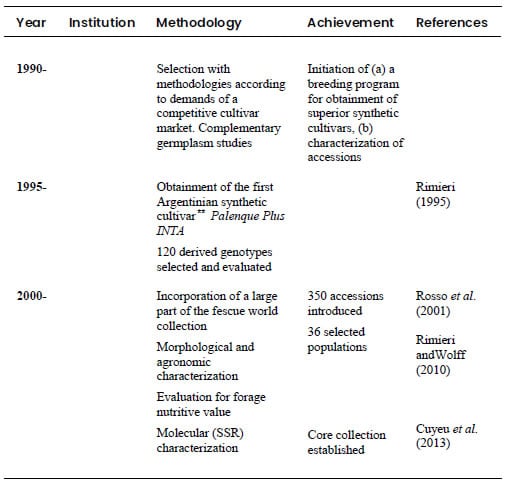
succinctly described in Table 1. It is proposed as an integral model for
germplasm management and utilization in general.
Table 1. Methods and
achievements in the Argentinian tall fescue (Festuca arundinacea Schreb.)
breeding program: from germplasm introduction and collection to obtainment of
commercial cultivars.
The needs of initiating a tall fescue breeding program and of creating a
forage germplasm bank in the country stepped from the following:
(1) agroecological conditions: (a) there were no native forage species adapted
to cattle grazing, and (b) the forage production of native and naturalized
forage species subjected to intensive grazing was low.
(2) technological situation: (a) there were no forage germplasm banks,
and (b) the grasslands were subjected to intensive grazing.
In response to this situation:
(1) Temperate forages species with high forage production and adapted to
intensive grazing were introduced, characterized and evaluated in agronomic,
biological, genetical and animal production studies.
(2) Populations and ecotypes for
planting and grazing were selected; cultivars were created, released and
disseminated in the region (the area of cultivated pastures was increased with
the local cultivar Pergamino El Palenque MAG); adaptation and production
were evaluated. This germplasm became part of both the working collection of
the forage breeding program and the germplasm bank of Pergamino Exp. Stn.
(3) Foreign cultivars were introduced to
widen the genetic base of tall fescue in Argentina but, in general, they had
poor agroecological and grazing adaptation.
(4) The need of exploring the available
global germplasm was established. A forage germplasm bank was created with the
adapted local germplasm and the world collection. Collections were evaluated
and characterized; protocols were applied to maintain the genetic diversity;
core collections were created.
(5) Pre-breeding was initiated for other
traits (adaptation to saline soils, forage nutritional value, etc.).
(6) Selected genotypes continued to be
incorporated into the germplasm bank.
(7) Animal production was
increased in the region. Summarizing, steps and protocols were followed in tall
fescue to integrate objectives of introduction of forage species for intensive
grazing, obtainment of populations and ecotypes for germplasm management and
utilization in integrated crop-livestock systems, adoption of modern cultivars,
pre-breeding for other traits, enhancement of the germplasm bank and increase
of animal production. We consider that the Argentinian tall fescue breeding
program is a good example of FAO´s proposition (FAO, 1996) on the association
and complementation of germplasm banks with breeding programs.
CONCLUSIONS
The premises of this paper are that ex situ conservation of the
genetic diversity contained in CWR and the utilization of the natural genetic
variability in cultivar breeding require the application of reproduction and population
genetics concepts in order to choose or develop the appropriate criteria and
experimental strategies. An important fact that needs to be taken into
consideration for devising germplasm collection and ex situ conservation
strategies is that the modes and types of reproduction have different genetic
consequences for the following generation. Natural or naturalized populations,
even those of autogamous species, can be heterogeneous, and the predominant
mode and type of reproduction of a given species can vary according to
environmental conditions during the growing cycle. Biological systems,
particularly plant systems, are very complex, thus, assumptions are usually
made in an attempt to investigate them. Since discrepancies between “reality”
and “assumptions” can be large, the conclusions withdrawn from experimental
works need to be adjusted to the plant materials and methods of study to have
scientific support. In this regard, there are many reports in the literature on
plant and crop physiology of the main food crops (e.g., wheat, maize,
sunflower, soybeans) and the “genetic progress” or “genetic gain” that has been
achieved in commercial cultivar breeding over the past decades (see Lo Valvo et al. 2018 as an example).
However, their potential contribution in crop breeding needs to be ascertained
by making focus on the analysis of the genetic structure of populations and the
sources of genetic variability available to the breeder (commercial cultivars,
land races, CWR). The genetic structure has to be related to the main methods
used in those studies and others of related disciplines for the interpretation
of the results in the frame of their eventual application in crop management or
breeding.
PROPOSAL
We consider that the
following information is needed as a basic input to start the analysis of the
current germplasm bank protocols at the light of the principles and methods of
Genetics: (a) Genus (or genera) and species of accessions in the germplasm bank
(b) Preponderant mode(s) and type(s) of reproduction (c) Geographic
distribution and sampled areas (d) Sampling strategies (e) Passport data of
collections in general, from the oldest to the newest (f) Ex situ regeneration/multiplication
protocols (g) Characterization type (morphological, genetic, molecular,
agronomic), if any. This information would allow the evaluation in the ex
situ collections of: (a) Representativeness of the collections,
geographical and environmental (at macro- and micro- levels). (b) Adequacy of
strategies and protocols for collection and regeneration or multiplication of
accessions to the principles of population genetics: population reproductive
size (N= actual number of plants in the population, and Ne=
effective number of plants, which contribute alleles to the next generation),
population genetic structure, gene (allele) frequencies, processes that can
alter gene frequencies. (c) Representativeness of the natural genetic diversity
in the collections. (d) Necessity of carrying out new collections in the
already sampled areas or in as yet unexplored ones. Furthermore, to ascertain
if wild germplasm conservation and commercial breeding converge at some point,
the following questions should be addressed: (1) In pre-breeding: (a) Is pre-breeding
an objective of germplasm banks? (b) What is considered to be more important in
the germplasm bank, the representativeness of the natural genetic diversity in
the accessions or the likely immediate use of the conserved germplasm? (2) In
breeding: (a) Is it considered that the collections can be directly used in
breeding programs or that pre-breeding is required as a first step? (b) Is it
known which is the genetic background of populations or genotypes adapted to
cultivation that has to be maintained or recovered after manipulations to
incorporate new germplasm in the cultivated pool (e. g. hybridizations,
backcrosses or other techniques or methods)? As a first step in this direction,
we will coordinate a workshop which is part of the program of ALAG 2021 (XVIII
Latin American Congress of Genetics; alagenet.
org/alag2021/en/scientific-program/#talleres). In advance, the invited
researchers and curators will provide in written response to the formulated
questions. The discussion and analysis of the responses will be carried out at
the light of the principles and methods of Genetics during the event. The
expected final product is a document on the current managing practices in
germplasm banks of seven participating countries; if appropriate, the document
will also contain propositions for the eventual modifications of protocols.
Finally, as Maxted and Kell (2009) have pointed out,
there is a need for CWR characterization and evaluation, development of genomic
databases of known useful genes from these sources, and improvement of gene
transfer techniques from wild to cultivated species, among others.
Notwithstanding, we consider that a previous basic requirement for successful
conservation and utilization of the natural genetic diversity and genetic
variability is the application of strategies and protocols based on the
principles and methods of population genetics, modes of reproduction and
genetic structures of CWR populations.
ACKNOWLEDGEMENTS
To former students and colleagues that, throughout the years,
contributed to the generation of knowledges and the discussion of ideas on
germplasm conservation and use. To the public institutions that provided
financial support for the authors´s lines of investigations and graduate
students´ scholarhips, mainly Instituto Nacional de Tecnología Agropecuaria
(INTA); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); Facultad
de Ciencias Agrarias, Universidad Nacional de Mar del Plata (UNMdP); and Facultad
de Ciencias Agrarias, Universidad Nacional de Rosario (UNR). To Dr. J. Federico
Maune, who kindly diagrammed Figure 1.
REFERENCES
Allard R.W. (1999) Principles of Plant Breeding (2nd ed.). John Wiley
& Sons, Hoboken, New Jersey.
Al-Khayri J.A., Jain S.M., Johnson D.V. (2015) Advances in Plant
Breeding Strategies: Breeding, Biotechnology and Molecular Tools, Vol. 1. Springer
International Publishing, Switzerland.
Asker S. (1980) Gametophytic apomixis: elements and genetic regulation. Hereditas
9: 277-293. Doi: 10.1111/j.1601-5223.1980.tb01367.x.
Bedonni M.C., Camadro E.L. (2009) Morphological and molecular evidence
of natural interspecific hybridization in the diploid potato Solanum
kurtzianum from Argentina. Botany 87: 78-87. Doi: 10.1139/B08- 116.
Boelcke O., Echeverría I. (1950) Valor
y comportamiento de mezclas forrajeras comerciales para praderas permanentes en
la región de Pergamino. Publicación No. 26.
INTA Estación Experimental Pergamino, Argentina.
Burnham C.R. (1980) Discussions in Cytogenetics (6th Ed.). University of
Minnesota, St. Paul.
Cadima Fuentes X., van Treuren R., Hoekstra R., van den Berg R.G., Sosef
M.S.M. (2017) Genetic diversity of Bolivian wild potato germplasm: changes
during ex situ conservation management and comparisons with resampled in
situ populations. Genet. Resour. Crop Evol. 64: 331-344. Doi: 10.1007/s10722-015-0357-9.
Camadro E.L. (2012) Relevance of the genetic structure of natural
populations, and sampling and classification approaches for conservation and
use of wild crop relatives: potato as an example. Botany 90: 1065-1072. Doi: 10.1139/b2012- 090.
Cardi T. (2016) Cisgenesis and genome editing: combining efforts for a
smarter use of genetic resources in crop breeding. Plant Breeding 135: 139- 147.
Doi: 10.1111/pbr.12345
CBD (2020) Convention on Biological Diversity. https://www.cbd.int
(accessed November 2020)
Cooper HD, Spillane C, Hodgkin T (2000) Broadening the
genetic base of crop production. CABI, Wallingford; FAO and IPGRI, Rome. http://www.bioversityinternational.org/Publications/pubfile.asp?ID_PUB=618.
(accessed June 2020).
Cuyeu A.R., Rosso B., Pagano E., Soto G., Fox R., Ayub N.D. (2013) Genetic
diversity in a world germplasm collection of tall fescue. Genet. Mol. Biol. 36:
237-242. Doi: 10.1590/S1415- 47572013005000021.
Dumas C., Mogensen H.L. (1993) Maize as a model system for experimental
embryogenesis in flowering plants. Plant Cell 5: 1337-1348. Doi: 10.1105/
tpc.5.10.1337.
Ellstrand N.C., Roose M.L. (1987) Patterns of genotypic diversity in
clonal plant species. Am. J. Bot. 74: 123-131. Doi: 10.1002/j.1537-2197.1987.tb08586.x.
Engels J.M.M., Visser L. (2006) Guide to effective management of
germplasm collections. IPGRI Handbooks for Germplasm Banks 6. IPGRI, Rome.
Erazzú L.E., Camadro E.L., Clausen A.M. (2009) Persistence over time,
overlapping distribution and molecular indications of interspecific hybridization in wild
potato populations of Northwest Argentina. Euphytica 168: 249-262. Doi: 10.1007/
s10681-009-9938-z.
Eriksson D., Ortiz R., Visser R.G.F., Vives- Vallés J.A., Prieto H. (2020)
Editorial: Leeway to operate with plant genetic resources. Front. Plant Sci 11:
911. Doi: 10.3389/fpls.2020.00911.
FAO (1996) The State of the World’s Plant Genetic Resources for Food and
Agriculture. FAO, Rome.
FAO (2017) Voluntary guidelines for the conservation and sustainable use
of crop wild relatives and wild food plants. FAO, Rome. http://www.fao.org/3/ai7788e.pdf.
(accessed June 2020).
Frankel O.H. (1984) Genetic perspectives of germplasm conservation. In: Arber
W., Llimensee K., Peacock W.J., Starlinger P. (Eds.) Genetic Manipulation:
Impact on Man and Society. Cambridge University Press, Cambridge.
Frankel R., Galun E. (1977) Pollination Mechanisms, Reproduction and Plant
Breeding. Springer-Verlag, Berlin, Heidelberg, New York.
Gallais A. (1990) Théorie de la sélection en amélioration des plantes. Masson,
Paris.
Gallais A., Bannerot H. (1992) Améloration des espéces végétales
cultivées. Objetives et critéers de sélection. INRA, Paris.
Gepts P. (2006) Plant Genetic Resources Conservation and Utilization:
The Accomplishments and Future of a Societal Insurance Policy. Crop Sci. 46: 2278-2292.
Doi: 10.2135/cropsci2006.03.0169gas.
Grant V. (1981) Plant Speciation. Columbia University Press, New York
and London.
Hammer K., Teklu Y. (2008) Plant Genetic Resources: Selected Issues from
Genetic Erosion to Genetic Engineering. J. Agric. Rural Dev. Trop. Subtrop. 109:
15-50.
Johnson R.C., Hodgkin T. (1999) Core collections for today and tomorrow. IPGRI, Rome
. http://www.bioversityinternational.org/publications/Web%5Fversion/43/.
(accessed June 2020).
Knox R.B. (1967) Apomixis: Seasonal and Population Differences in a
Grass. Science 157: 325-326. Doi: 10.1126/science.157.3786.325.
Langer R.H.M., Wilson D. (1965). Environmental control of cleistogamy in
prairie grass (Bromus unioloides H.B.K.). New Phytol. 64: 80-85. Doi: 10.1111/j.1469-8137.1965.tb05377.
Leofanti G.A., Camadro E.L., Erazzú L.E. (2019) Variation over time in
morphological phenotypes and reproductive behavior in a natural wild potato
population from Tucumán, Argentina. Genet. Resour. Crop
Evol. 67: 139-161. Doi: 10.1007/s10722-019-00858-7.
Leofanti G.A., Camadro E.L., Echeverría M.M., Alonso S.I. (2013) Anormalidades meióticas en especies
nativas del género Bromus (Secc. Ceratochloa) de la
Argentina. BAG. J. Basic Appl. Genet. 23 (Suppl. 1): 78.
Lo Valvo P.J., Miralles D.J., Serrago R.A. (2018) Genetic Progress in
Argentine bread wheat varieties release between 1918 and 2011: Changes in
physiological and numerical yield components. Field Crop Res. 21: 314-321. Doi:
10.1016/bs.agron.2021.02.005.
Maddaloni J., Ferrari L. (2001) Forrajeras
y pasturas del ecosistema templado húmedo de la Argentina. Facultad de Cs.
Agrarias, Universidad Nacional de Lomas de Zamora, Lomas de Zamora.
Maxted N., Kell S. (2009) Establishment of a Global Network for the Ex
Situ Conservation of Crop Wild Relatives: Status and Needs. FAO Commission on
Genetic Resources for Food and Agriculture. FAO, Rome .
Maxted N., Ford-Lloyd B.V., Hawkes J.G. (1997) Complementary
Conservation Strategies. In: Maxted N., Ford-Lloyd B.V., Hawkes J.G. (Eds.) Plant
Genetic Conservation: The In Situ Approach. Chapman and Hall, London, pp. 15-40.
Maxted N., Castañeda Álvarez N.P., Vincent H.A., Magos Brehm J. (2012) Gap
analysis: a tool for genetic conservation. In: Guarino L., Ramanatha Rao V., Goldberg
E. (Eds.) Collecting Plant Genetic Diversity: Technical Guidelines, 2011 update.
Biodiversity International, Rome, pp. 1-17.
Maxted N., Amri A., Castañeda-Alvarez N.P., Dias S., Dullo M.E., Fielder
H., Ford-Lloyd B. V., Iriondo J.M., Magos Brehm J., Nilsen L.B., Thormann I., Vincent
H., Kell S.P. (2016) Joining up the dots: a systematic perspective of crop wild
relative conservation and use. In: Maxted N. et al. (Eds.) Enhancing crop gene
pool use: capturing wild relative and landrace diversity for crop improvement. Cambridge University Press, Cambridge , pp. 87-124.
Poulsen Hornum A., Camadro E.L. (2021) Expression of internal
reproductive barriers in a germplasm bank accession of the wild potato Solanum
chacoense Bitter in three ex situ regeneration cycles. Genet. Resour. Crop Evol. 68:915-938. Doi: 10.1007/s10722-020-
01034-y.
Quarin C.L. (1986) Seasonal changes in the incidence of apomixis of
diploid, triploid, and tetraploid Paspalum cromyorrhizon. Euphytica 35: 515-522. Doi: 10.1007/BF00021860.
Rebozzio R., Sartor M., Quarin C.L., Espinoza F. (2011) Residual
sexuality and its seasonal variation in natural apomictic Paspalum notatum accessions.
Biol. Plantarum 55: 391- 395. Doi: 10.1007/s10535-011-0062-2.
Rieger R., Michaelis A., Green M.M. (1976) A Glossary of Genetics,
Classical and Molecular (5th ed.). Springer-Verlag, Berlin , Heidelberg, New York.
Rimieri P. (1995) Palenque Plus INTA. Expte. INASE No. 3780 (05/29/95) https://inta.gob.ar/variedades/palenque-plus-inta.
(accessed June
2020).
Rimieri P. (2017) La diversidad
genética y la variabilidad genética: dos conceptos diferentes asociados al
germoplasma y al mejoramiento genético vegetal. BAG. J. Basic
Appl. Genet. 28 (2): 7-13.
Rimieri P., Wolff R. (2010) La
genética y el estado actual de la obtención y adopción de cultivares forrajeros
en Argentina. BAG. J. Basic Appl. Genet. 1 (2): 1-7.
Rosso B., Pagano E., Rimieri P. (2001) Evaluation and utilization of the
tall fescue germplasm collection at Pergamino INTA, Argentina. Proceedings of
the XIX International Grassland Congress. Sao Paulo, Brazil, p 504. https://www.internationalgrasslands.org/publications.
(accessed July 2020).
Schoen D.J., Brown A.H.D. (1991) Intraspecific variation in
population gene diversity and effective population size correlates with the
mating system in plants. P. Natl. Acad. Sci. USA 88: 4494-4497. Doi: 10.1073/
pnas.88.10.4494.
Serrano H. (1985) Premio
Academia Nacional de Agronomía y Veterinaria. http://bibliotecadigital.bolsadecereales.com.ar/greenstone/collect/pubper/index/assoc/HASH1b0b/d2948809.dir/Ejercicio%201986.pdf.
(accessed July
2020)
Sirakaya A. (2019) Balanced Options for Access and Benefit-Sharing:
Stakeholder Insights on Provider Country Legislation. Front. Plant Sci.
10: 1175. Doi: 10.3389/fpls.2019.01175.
UPOV (2002) Council Nineteenth Extraordinary Session Geneva, April 19,
2002. https://www.upov.int/edocs/infdocs/en/0_c_extr_19_2_rev.pdf. (accessed August
2020).
UPOV (2016) Symposium on possible interrelations between the
International Treaty on Plant Genetic Resources for Food and Agriculture
(ITPGRFA) and the International Convention for the Protection of New Varieties
of Plants (UPOV Convention). https://www.upov.int/edocs/mdocs/upov/en/upov_itpgrfa_sym_ge_16/upov_itpgrfa_sym_ge_16_2_proceedings.pdf.
(accessed August 2020).
Villar A.D., Serrano H. (1963) Praderas
permanentes para la región pampeana húmeda. Boletín Técnico No. 2, INTA Rafaela Experimental
Station, Rafaela.
Visser B., Nap J.P. (2002) Biotechnology and agrobiodiversity in
Biological and Medical Sciences. Encyclopedia of Life Support Systems (EOLSS). Eolss
Publishers, Oxford. http://www.eolss.net
Williams W.M., Stewart A.V., Williamson M.L. (2011) Bromus. In: Kole
C. (Ed.) Wild Crop Relatives: Genomic and Breeding Resources, Millets and
Grasses. Springer Verlag, Berlin, pp. 15-30.
Wolff R., Abbott L., Pistorale S. (1996) Reproductive behavior of Bromus
catharticus Vahl. (Cebadilla criolla) in natural and cultivated populations.
J Genet Breed 50: 121-128.



